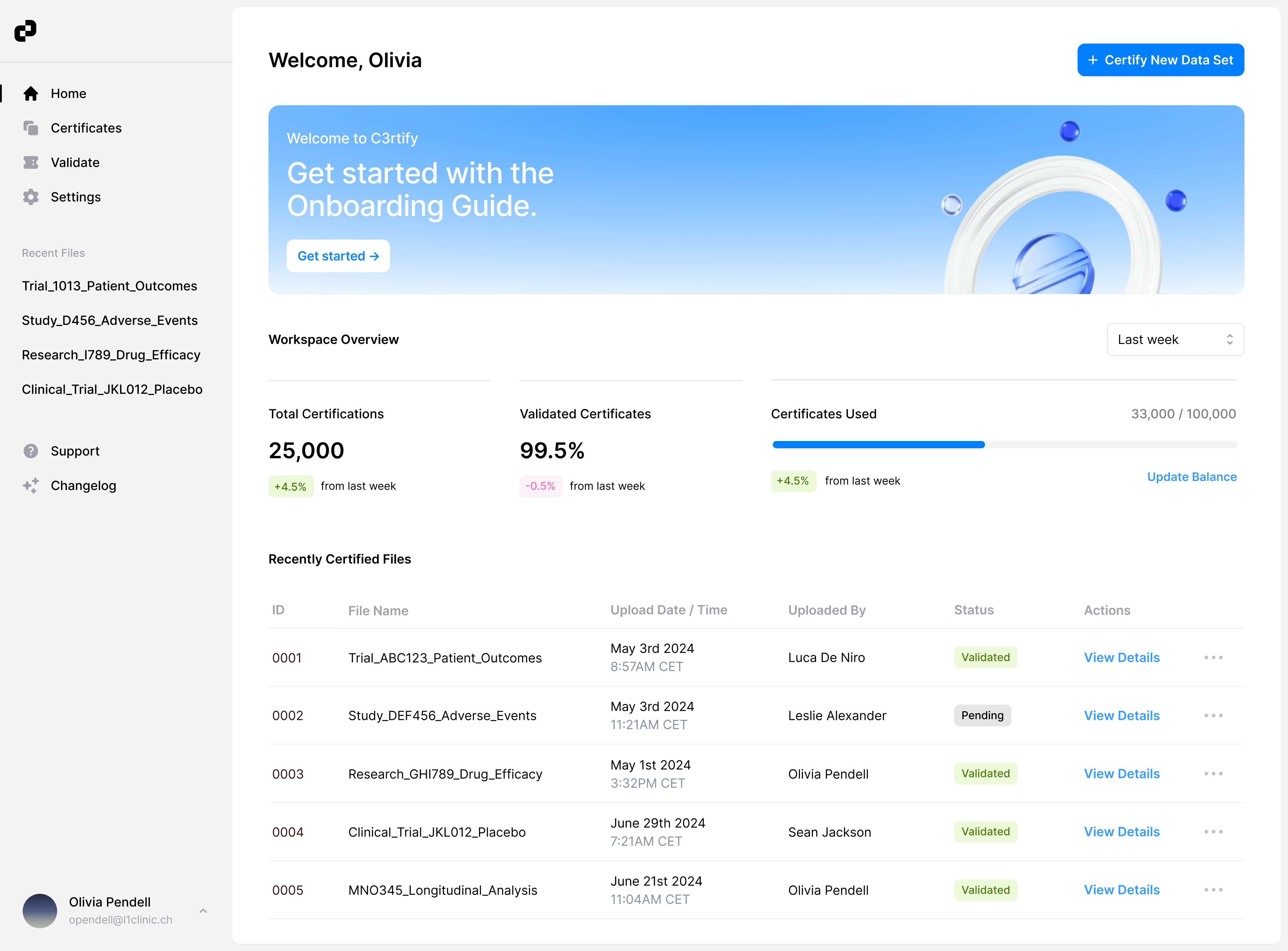
Setup and configure your organisation in minutes
Easily onboard your team and manage data efficiently with organisation controls.
Achieve effortless compliance and maintain audit-ready data
Ensure audit compliance instantly with automated checks that verify data integrity and confirm no alterations.

Protect patient data with state of the art security and compliance
Meet stringent regulatory standards, ensuring absolute data integrity across trials.

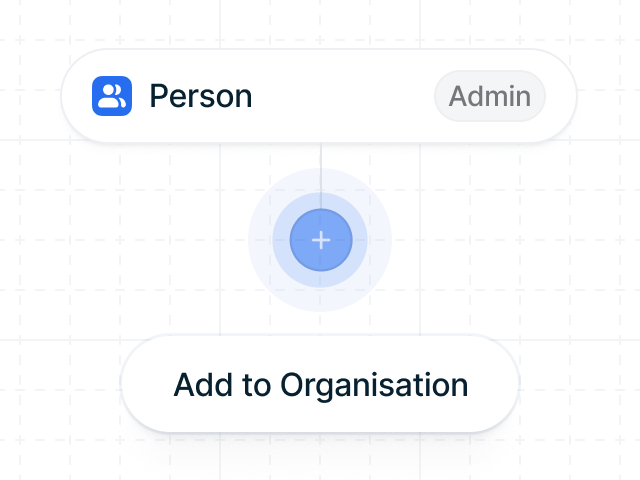
Sensitive data protected with Zero-Knowledge Proofs
Guarantee data authenticity and integrity without exposing any sensitive information.
Upload your data securely and effortlessly
Choose the most convenient method to seamlessly and securely import your data.

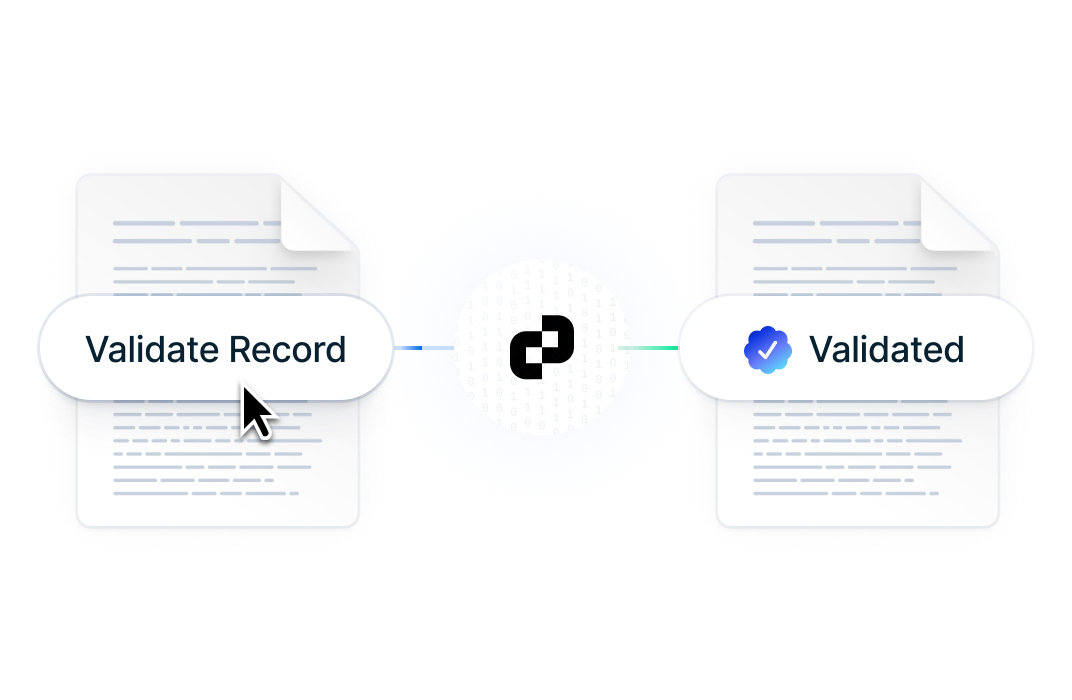
Verify your data's authenticity with a single click
Instantly validate data integrity to confirm that no information has been tampered with.
Start validating your clinical trials in minutes. Store your records in just a few easy steps.
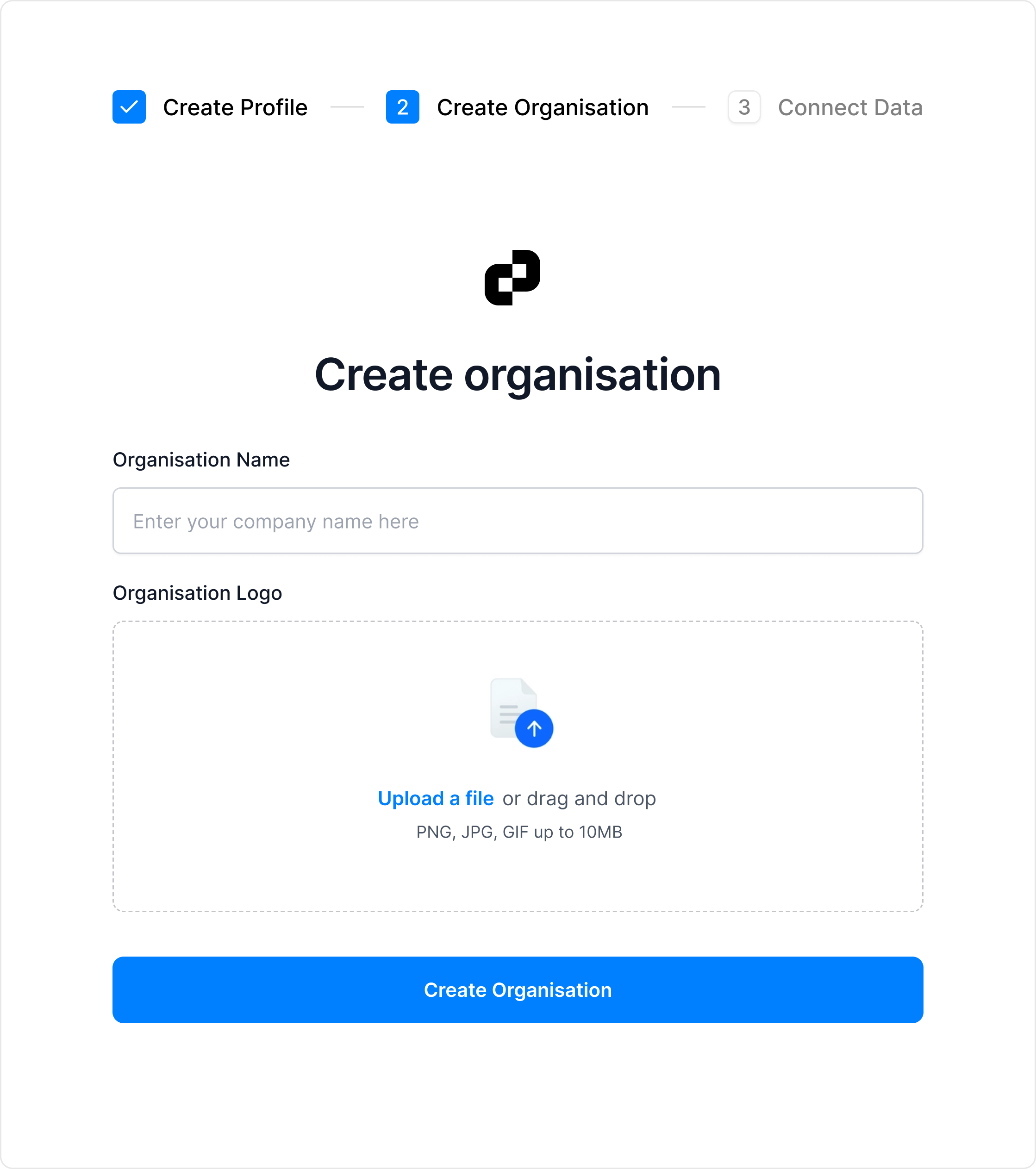
Easily Onboard and Manage Teams
Get started with a simple onboarding —create your user profile and organization account. With organizations, you can easily view, manage, and validate multiple data sets.
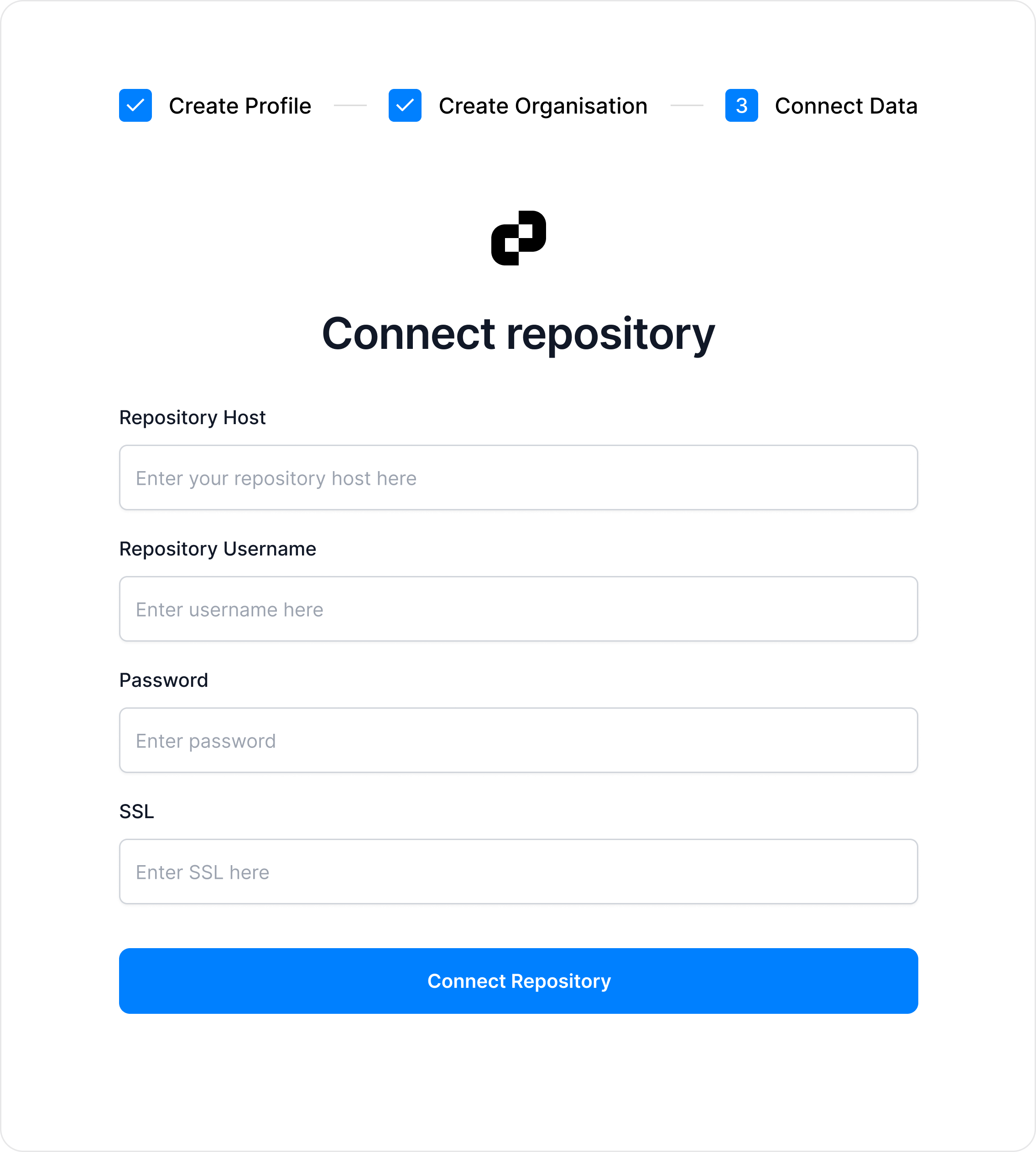
Connect your own repository
Link your data securely in minutes. All sensitive information remains in your repository, while only cryptographic hashes are stored on the blockchain for verification.
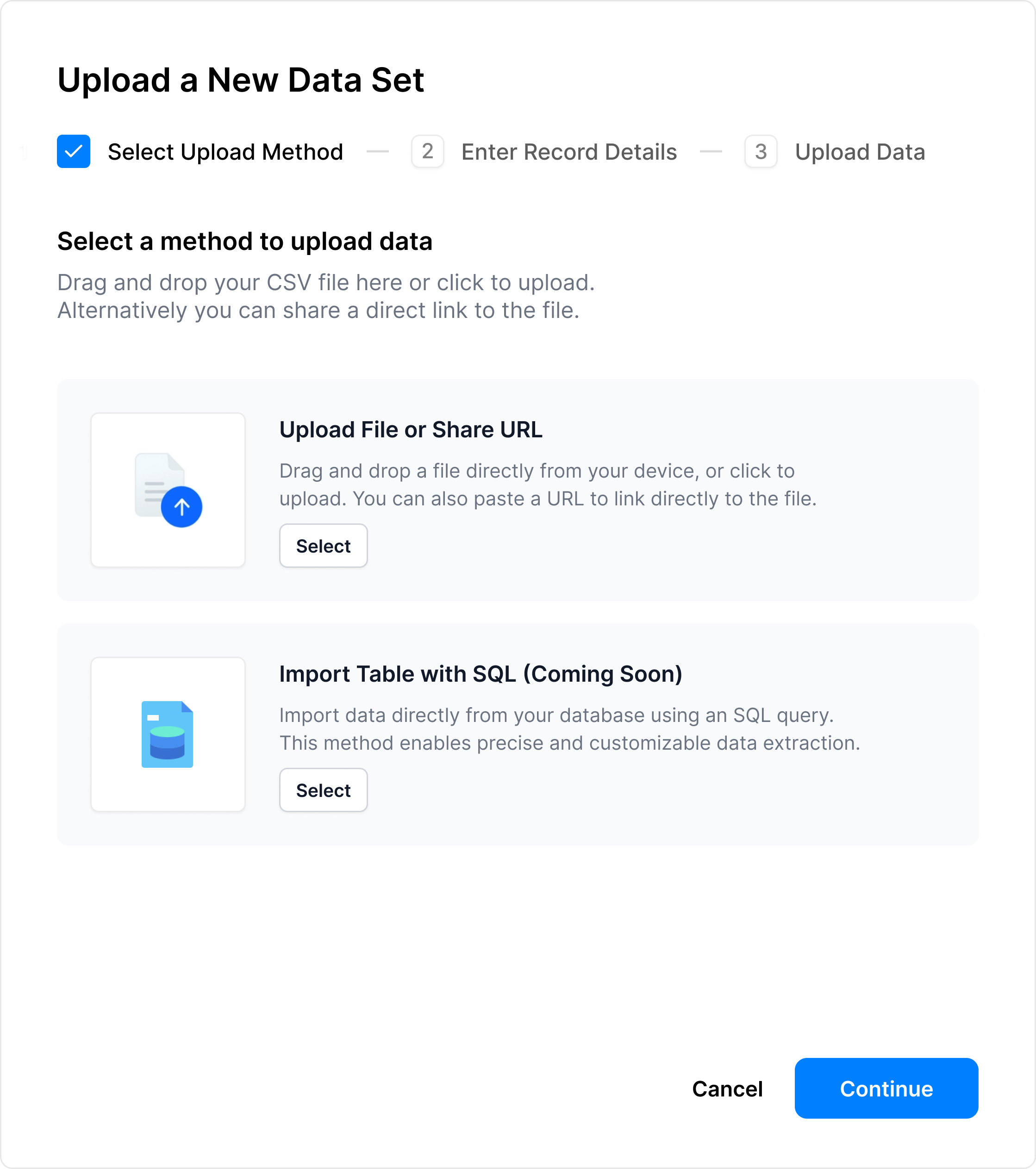
Upload a data set
Upload your dataset in just a few clicks: choose direct upload, import via URL, or connect through a SQL query.
View and validate your certificate
Quickly verify the integrity of your clinical trial data. Our system secures and monitors your data, providing real-time confirmation of authenticity and peace of mind.
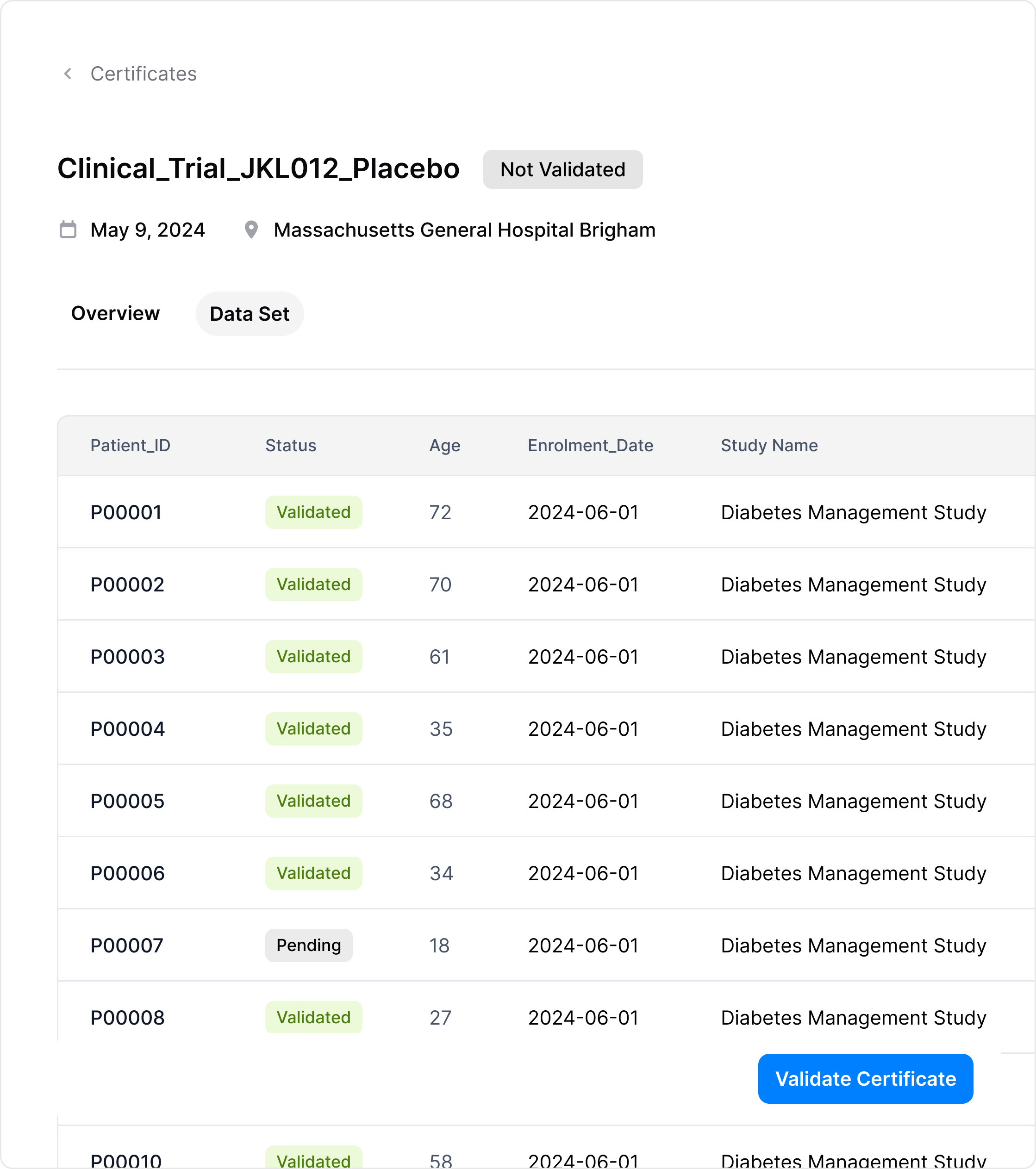
Store and Revalidate Certificates Anytime, Anywhere
Securely store your certificates for easy access, and revalidate them as needed to ensure data integrity and confirm that your information remains intact and unchanged.
Reduce Operational Costs and Streamline Trial Management
Discover how C3rtify addresses key challenges in healthcare.
Discover how C3rtify tackles critical healthcare challenges.
Discover how C3rtify addresses key challenges in healthcare.
Frequently Asked Questions
Everything you need to know